Summary
Background: A 62-year-old male kidney transplant recipient was admitted to hospital with a 14-day history of fever, hemoptysis and left-sided pleuritic chest pain. He had suffered malaise, weight loss, night sweats and exertional dyspnea over the previous 3 months. Imaging studies of the patient's chest revealed a noncavitated mass measuring 5 × 8 cm in the anterior segment of the left upper lobe of the lung and a left-sided pleural effusion with septa, and bacterial cultures revealed the presence of Rhodococcus equi.
Investigations: Physical examination, laboratory tests, chest X-ray, CT scan of the chest, bronchoscopy, and bacterial culture of blood, sputum, bronchoalveolar lavage fluid and pleural fluid.
Diagnosis: R. equi infection with bacteremic pleuropneumonia and pseudotumor. A secondary myopathy occurred 6 months after diagnosis of the infection as a result of a drug interaction between clarithromycin and simvastatin.
Management: Long-term combination antibiotic therapy (ciprofloxacin plus vancomycin or clarithromycin), resection of the inflammatory pseudotumor, and reduction of immunosuppressive therapy. Following the diagnosis of myopathy, simvastatin was discontinued.
The Case
A 62-year-old man was admitted to hospital with a 14-day history of fever, hemoptysis and left-sided pleuritic chest pain. Over the previous 3 months he had suffered B symptoms (night sweats and 7 kg weight loss) accompanied by progressive dyspnea on exertion and general malaise.
The patient had undergone renal transplantation at the age of 43 years because of end-stage renal disease secondary to IgA nephropathy. The patient's initial post-transplantation immunosuppression regimen had consisted of prednisone and ciclosporin A. One year after transplantation, azathioprine had been added to the regimen, with a concomitant reduction in ciclosporin A dose because of the development of gingival hyperplasia. Seventeen years after the transplantation, the patient had been started on triple therapy with low-dose ciclosporin A (target trough level 60 μg/l), mycophenolate sodium (Myfortic®; Novartis AG, Basel, Switzerland) and prednisone in place of the original regimen because of histologically proven chronic allograft nephropathy. The patient was originally from Bosnia but had lived in a rural area in Switzerland for more than 20 years. He had worked in a slaughterhouse until he was 55 years old. He had never smoked.
On admission to hospital with fever, hemoptysis and left-sided pleuritic chest pain, the patient seemed to be chronically ill. He was febrile and somnolent, but he was eupneic and hemodynamically stable. The patient's renal transplant function had deteriorated and he had an estimated creatinine clearance of 30 ml/min (previously 40–50 ml/min following transplantation), a C-reactive protein (CRP) level of 220 mg/l (normal range <8 mg/l), and a white blood cell count of 12 × 109/l. On admission, the patient was on prednisone 7.5 mg/day, Myfortic® 540 mg twice daily, ciclosporin A 50 mg twice daily (target trough level 60 μg/l), simvastatin 40 mg/day, aspirin 100 mg/day, esomeprazole 20 mg/day, various antihypertensive drugs, vitamin D and alendronic acid 70 mg/week.
A chest X-ray revealed that the patient had an enlarged left hilus and a left-sided pleural effusion. A CT scan of the chest revealed a contrast-enhanced noncavitated mass measuring 5 × 8 cm in the anterior segment of the left upper lobe of the lung and a left-sided pleural effusion with septa (Figure 1). Empiric therapy for pleuropneumonia was started (intravenous amoxicillin–clavulanate potassium 1.2 g three times a day) and the Myfortic® dose was reduced to 360 mg twice daily. The patient underwent an HIV test, the results of which were negative. No amelioration in the patient's symptoms was observed with the new treatment; therefore, further diagnostic procedures were undertaken.
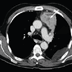 | Figure 1. (click image to zoom) A CT scan of the chest. The image shows a 5 × 8 cm mass (arrow) in the anterior segment of the left upper lobe and a left-sided pleural effusion with septa. |
On the patient's admission to hospital, bronchoscopy findings were macroscopically normal and no mycobacteria were detected in his sputum samples, bronchoalveolar lavage fluid or pleural fluid. One week after his admission, cultures from these samples and from blood samples returned positive for Rhodococcus equi.
Following an investigation of the literature for examples of treatment strategies for R. equi infection in humans, and according to the in vitro minimal inhibitory concentration of the drugs against R. equi strains in this case, the patient's physicians changed his treatment regimen to intravenous vancomycin (target trough level 10–20 mg/l) and oral ciprofloxacin 500 mg twice daily. The patient's fever subsided within several days, but 4 weeks later his sputum was still positive for R. equi, his CRP level was still high (70 mg/l) and a CT scan showed that there was no reduction in the size of the pulmonary mass. A percutaneous biopsy of the pulmonary mass was inconclusive. In addition, a post-biopsy complication of a fistula with subcutaneous emphysema occurred. For this reason, and also to decrease the microbial burden and to exclude neoplasia, resection of the pseudotumor and thickened pleura was performed (Figure 2). Histological examination of the resected lung tissue revealed fibrous pleuritis, as well as perivascular granulomas composed of spindle cells and epithelioid cells. Gram staining revealed Gram-positive cocci within the cytoplasm of inflammatory cells (Figure 3).
 | Figure 3. (click image to zoom) Histology of the resected lung tissue with Gram staining. The image shows Gram-positive cocci within the cytoplasm of inflammatory cells. |
Oral ciprofloxacin (500 mg twice daily) and intravenous vancomycin (target trough level 10–20 mg/l) were continued. On discharge (on the 13th postoperative day), the patient's CRP level was 9 mg/l and his creatinine clearance had increased to 44 ml/min.
Three months after the patient's discharge from hospital, clinical and radiological remission of his R. equi infection was confirmed and the antibiotic treatment regimen was simplified by a switch to ciprofloxacin (500 mg twice daily) and clarithromycin (250 mg three times a day, adjusted according to renal function) so that no further parenteral medication was necessary. The ciclosporin A dose was reduced under intensive monitoring of the trough levels, the aim being a stable trough level of 60 μg/l. The dose of Myfortic® was increased back to 540 mg twice daily.
After 3 months of treatment with clarithromycin and ciprofloxacin, the patient was readmitted to hospital with subacute bilateral proximal paraparesis. On physical examination, the patient was unable to stand up from a chair without the support of his arms. He had no muscle pain and no other neurological deficits. His creatine kinase level was elevated (2,976 U/l; normal <170 U/l). Electroneuromyography of the proximal leg muscles showed findings consistent with myopathy. The most probable cause of myopathy was simvastatin, as this drug had not been discontinued when the patient was started on clarithromycin. After discontinuation of simvastatin, the patient's muscle strength improved, and his creatine kinase level returned to normal within 1 week.
After completing a total of 9 months of antibiotic treatment, the patient remained in remission from R. equi infection.
Discussion of Diagnosis
The case presented here illustrates the importance of extensive diagnostic procedures in kidney transplant recipients. The clinical presentation of a disease can be atypical in immunocompromised patients, and the spectrum of potential pathologies (e.g. carcinomas) and possible disease-causing microbial agents (e.g. viruses, fungi, and bacteria such as Mycobacterium tuberculosis) in such patients is large. Continued investigations are, therefore, warranted until a definite diagnosis can be made.
R. equi was first isolated from foals in 1923 as a cause of pyogranulomatous pneumonia, and can be found in soil and in the manure of herbivores worldwide. In humans, R. equi infection can be acquired by inhalation, ingestion, or direct inoculation into a wound. R. equi infection usually affects humans who have impaired cellular immunity, so its incidence in humans has increased markedly over the past few decades as a result of higher numbers of organ transplantations, increased use of chemotherapy for malignancies, and increases in the incidence of HIV infection.[1,2] The patient presented here could not recall any contact with horses or other domestic animals since he had retired at the age of 55 years, and he denied any contact with soil (e.g. through gardening).
R. equi is a Gram-positive, aerobic bacterium that can exist as a coccus or a bacillus and is sometimes acid-fast. Pale salmon-pink colonies appear on blood agar after 4–7 days of incubation. R. equi is a facultative intracellular pathogen of macrophages; its capacity to survive inside histiocytes enables chronic infection.[1,2]
Histopathology of biopsy specimens infected with R. equi usually reveals a necrotizing granulomatous pneumonia (as in the case presented here), with inflammatory pseudotumors, intracellular Gram-positive cocci, and granulomatous and fibrinous pleuritis.[1,2]
Approximately 80% of humans infected with R. equi develop a pulmonary infection such as pneumonia.[1] The infection can be complicated by nodules (e.g. pseudotumors) and/or cavities, which each usually occur in the upper lobes of the lungs. Other complications include pleural effusion, empyema, and invasion into the contiguous chest wall. Onset of the pneumonic presentation of R. equi infection is typically subacute with fever, cough and fatigue. Relapses are common and frequently occur at extrapulmonary sites such as the brain or as subcutaneous abscesses. Although rare, relapses can also occur as abscesses in the liver, spleen, thyroid, kidney, psoas muscle, bone, prostate, intra-abdominal cavity or paraspinous tissue. Extrapulmonary R. equi infections described in humans include wound infections, traumatic septic arthritis, and endophthalmitis following ocular injury.[1,2]
The best method of diagnosis of R. equi infection is the isolation of the bacteria in cultures from the infection site (e.g. sputum sample, bronchial brushing samples, bronchoalveolar lavage fluid, fine-needle aspiration biopsy sample or open lung biopsy sample).1 In the patient presented here, the API Coryne system (bioMérieux, l'Etoile, France) was used to identify R. equi in cultures from blood, sputum, bronchial fluid and pleural fluid. Bacteremia, as was present in the patient presented here, occurs in 25% of solid organ transplant recipients with R. equi infection.[1]
The differential diagnosis of pulmonary R. equi infection is diverse (Box 1) because of the variable acid-fast nature and the diverse clinical presentation of the infection.
Treatment and Management
A MEDLINE search at the time of writing of this article revealed 20 cases of pulmonary R. equi infection in renal transplant recipients.[3-12] Treatment for R. equi infection has not been standardized as this opportunistic pathogen causes only sporadic infections in humans. Use of synergistic antibiotics with high intracellular penetration is recommended, however, owing to the intracellular location of the bacteria. Vancomycin, clarithromycin, rifampicin, ciprofloxacin and imipenem have all been used with success to treat R. equi infection.[1-12] Transplant recipients who are on calcineurin inhibitors should not, however, be given rifampicin or clarithromycin as a first-line treatment because of potential drug interactions. After the present case's physicians had studied previous case descriptions and consulted Mandell et al.'s Principles and Practice of Infectious Diseases,[1,3-12] the patient was administered combination antibiotic therapy with ciprofloxacin plus vancomycin or clarithromycin, for a total of 9 months to avoid relapse. Ciprofloxacin was selected for its bactericidal effect, its good intracellular penetration and the fact that it has little effect on ciclosporin A blood level. Therapy for R. equi infection should initially include an intravenous bactericidal drug; intravenous vancomycin was selected in this case, as successful use of this drug had been reported in the majority of cases described previously. The switch from intravenous vancomycin to oral clarithromycin (which also has good intracellular penetration and bactericidal action) required a reduction of the ciclosporin A dose with intensive monitoring of blood trough levels of ciclosporin A. To avoid relapse, treatment was scheduled to continue for at least 9 months, as with treatment of M. tuberculosis, which has similar microbiological characteristics to R. equi (especially intracellularity).[1]
Only a few publications state the size of the lung mass in renal transplant recipients with R. equi infection. Three patients were reported to have a lung mass that was similar in size to that in the patient presented here; two of these masses resolved without the need for surgery.[9-11] One patient whose mass resolved without surgical intervention had discontinued immunosuppression following a deterioration of her transplant function that had resulted in a need for hemodialysis.[9] The other patient had an M. tuberculosis infection, and a CT scan showed a reduction in the size of the mass following antimicrobial treatment for tuberculosis (ethambutol and pyrazinamide). Since a concomitant R. equi infection was diagnosed subsequently, therapy with imipenem, clarithromycin and ciprofloxacin for 15 days was added.[10]
As immunosuppression is a major predisposing factor for R. equi infection, there is a consensus that immunosuppressive therapy should be reduced in patients with R. equi infection who are on such drugs.
In the patient presented here, the decision to perform surgery was made because, after 1 month of antibiotic treatment and reduced immunosuppression, systemic inflammation had persisted, the sputum remained positive for R. equi and the pulmonary mass had not changed in size. The aim of the surgery was to remove the pseudotumor, thus reducing the microbial burden and also excluding the presence of neoplasia or an abscess.
The myopathy was an iatrogenic complication that was probably caused by an interaction of the actions of simvastatin, clarithromycin and ciclosporin A on the cytochrome P450 3A4 enzyme, which has an important role in drug metabolism in the liver. Simvastatin is more lipophilic than either pravastatin or fluvastatin, and it therefore has a very low bioavailability. Simvastatin undergoes high first-pass metabolism by cytochrome P450 3A4 in the liver, making it susceptible to potent cytochrome P450 3A4 inhibitors; in the presence of cytochrome P450 3A4 inhibitors, the first-pass metabolism of simvastatin is inhibited, leading to a marked increase in the concentration of this drug in the blood. Such increases might cause toxic effects on skeletal muscle. Clarithromycin acts as an irreversible and dose-independent inhibitor of cytochrome P450 3A4. If a patient already on simvastatin starts clarithromycin, myopathy due to an augmented blood level of simvastatin can develop at any time. If a patient on clarithromycin and simvastatin develops muscle pain or tenderness, statin therapy should be discontinued immediately and the patient's creatine kinase level should be measured. Ciclosporin A given concomitantly with simvastatin therapy is known to increase the area under the curve of simvastatin by eight times, through competition for drug-binding sites at the cytochrome P450 3A4 enzyme.[13-15]
In hindsight, the patient presented here should not have been administered a 40 mg/day dose of simvastatin in combination with ciclosporin A treatment. A lower dose of simvastatin should have been used, or simvastatin switched for the more-hydrophilic pravastatin or fluvastatin, with which the risk of myopathy is lower. In this patient, simvastatin should have been discontinued when clarithromycin was administered.
In general, statins—at reduced doses—can be used safely with ciclosporin A. The addition of a third agent that is also metabolized by the cytochrome P450 3A4 enzyme (e.g. clarithromycin, as in this case), however, increases the risk of statinrelated myopathy. The risk of statin-related myopathy is also increased in patients who have a creatinine clearance of less than 30 ml/min, those of advanced age, those with a small body frame, and those who are on medications such as fibrates, nicotinic acid, azole antifungals, protease inhibitors, nondihydropyridine calcium channel blockers or amiodarone.[13-15]
Conclusions
R. equi infection is a rare opportunistic infection in renal transplant recipients. Clinical, microbiological and therapeutic aspects of R. equi infection are similar to those of M. tuberculosis infection. This Case Study described a kidney transplant recipient who presented with pleuropneumonia and a large, solid pulmonary mass. Antibiotic therapy—initially ciprofloxacin and vancomycin—was administered. After 1 month, however, inflammation was not under control and a thoracotomy with resection of the pseudotumor was performed to reduce the microbial burden and exclude the presence of a neoplasm. Three months after surgery, the treatment regimen was simplified to oral ciprofloxacin and clarithromycin, with an aim of providing a total of 9 months of antibiotic therapy. This Case Study, which also demonstrates an iatrogenic complication of statin-induced myopathy, highlights the importance of considering all medications a patient is taking when administering new drugs, as immunosuppressive drugs are not the only medications involved in drug interactions.
Acknowledgments
The authors would like to thank C Öhlschlegel, K Boggian, W Nagel and W Riesen, all at Cantonal Hospital St Gallen, for their helpful input into this Case Study. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.